Disease of the Month – Pancreatic Cancer
Shots
- To keep our readers acquainted with several disease conditions, ongoing trials, and available treatment options, PharmaShots brings every month a detailed take on a particular disease after thorough research
- Continuing the series for the Disease of the Month, PharmaShots brings a condensed report on Pancreatic Cancer
- November 16th is observed as World Pancreatic Cancer Day. It remains fourth leading cause of cancer death in both men and women
Introduction1,2
Cancer that starts as a proliferation of cells in the pancreas is known as pancreatic cancer. It produces hormones to help control blood sugar levels and enzymes to aid in food digestion. Rarely is pancreatic cancer discovered when it has the best chance of being cured. This is because, in many cases, symptoms do not appear until the disease has progressed to other organs. Currently, the only treatment available is surgical resection. However, only 20% of cases with pancreatic cancer can be surgically resected at the time of diagnosis.
Symptoms1
Often, pancreatic cancer is symptomless until it has progressed. Signs and symptoms of pancreatic cancer may include: –
- Belly pain that spreads to the sides or back
- Loss of appetite
- Weight loss
- Jaundice (Yellowing of the skin and the whites of the eyes)
- Light-colored or floating stools
- Dark-colored urine
- Itching
- New diagnosis of diabetes or diabetes that’s getting harder to control
- Pain and swelling in an arm or leg, which might be caused by a blood clot
- Tiredness or weakness
Diagnosis1
Early detection of pancreatic cancer is challenging since regular checks are unable to detect the cancer, making it challenging to identify the tumors on normal imaging tests.
The combination of tests for the detection of pancreatic cancer are as follows:
- Imaging tests: These include CT scans, MRI, PET, and endoscopic ultrasound (EUS).
- Blood tests: Blood tests can detect tumor maker, a substance found during pancreatic cancer. Increased levels of carbohydrate antigen (CA) 19-9, a type of protein released by pancreatic cancer cells, indicate a tumor
- Staging Laparoscopy: A long tube is inserted through a tiny in the abdomen that has a camera at the end of it. This helps in identifying the anomalies in the abdomen
Epidemiology3
The American Cancer Society estimates that in 2023, ~64,050 people (33,130 men and 30,920 women) will be diagnosed with pancreatic cancer and ~50,550 (26,620 men and 23,930 women) deaths will occur in the US. It commonly affects men more than women.
Approximately 3% of cancer cases in the US are attributed to pancreatic cancer, contributing to around 7% of all cancer-related fatalities.
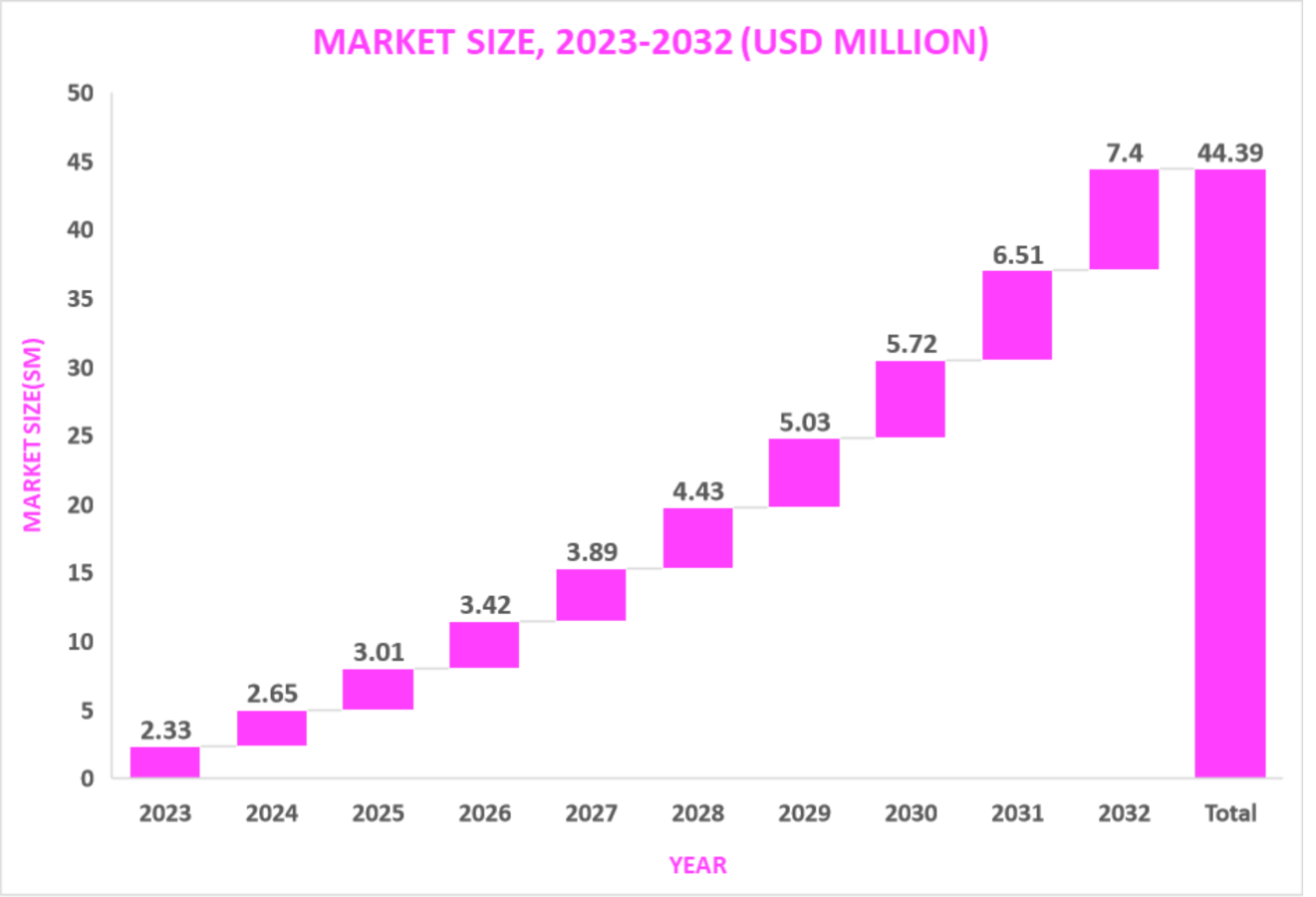
Market Size9,5
In 2022, the pancreatic cancer treatment market size reached $2.05B, and within 10 years (by 2032) it is expected to increase by $7.40B at a CAGR of 13.7%.
Management and treatment1
Surgery
Despite low survival rates, pancreatic cancer can be completely cured with prompt diagnosis and care. Complete surgical excision of malignancy is the only feasible treatment for pancreatic cancer.
- Whipple procedure (pancreaticoduodenectomy): Whipple procedure is suggested when tumor is in the head of the pancreas. This surgical procedure involves the removal of the head of the pancreas, duodenum, gallbladder, a portion of the bile duct, and nearby lymph nodes. The surgeon then reconnects the remaining pancreas and bile duct to the small intestine, restoring the digestive tract.
- Distal pancreatectomy: A distal pancreatectomy can be carried out by a surgeon if the tumor is located at the tail of the pancreas. A portion of the pancreas’ body as well as its tail is removed during this treatment
- Total pancreatectomy: In cases where cancer has spread throughout the entire pancreas but is still resectable, a total pancreatectomy may be considered. This surgery involves removing the entire pancreas, gallbladder, spleen, and a portion of the stomach and small intestine
Chemotherapy
Chemotherapy, as pills are administered through an intravenous route in the arm, are used to destroy tumor cells.
Radiation therapy
Radiation therapy, utilizing high-energy X-rays, is a common treatment for pancreatic cancer. Typically combined with chemotherapy (chemoradiation), it may be administered before or after surgery, or as a primary cancer treatment. For advanced pancreatic cancer cases where surgery isn’t an option, radiation therapy can help alleviate symptoms.
Targeted therapy
Targeted therapy employs drugs that target specific proteins controlling cancer cell growth and spread. It is often combined with other treatments such as radiation therapy
Pain management
Pancreatic cancer, often causing significant pain due to involvement with nearby nerves, can be managed by healthcare providers using oral medications, anesthesia, or steroid injections.
Product Dashboard

Key Players in the Market4, 6, 7

* The above-mentioned table depicts drug approval dates in descending order of the US
Clinical Trial Analysis8
Various pharmaceutical companies are working to develop new treatment options, either as monotherapy or in combination for pancreatic cancer. As of December 08, 2023, about 1133 interventional clinical trials have been registered worldwide for pancreatic cancer. Some of the key molecules involved in the trials are L-DOS47 + doxorubicin (Helix BioPharma and Theradex), U87 CART (Shanghai Unicar-Therapy Bio-medicine Technology) and IN10018 (InxMed)
Based on geographical distribution, the interventional and Industry sponsored clinical trials are classified in the below-mentioned graph in two groups based on their status i.e., active (recruiting, active, not recruiting, not yet recruiting and enrolling by invitation, suspended) and inactive (withdrawn, terminated and trials with unknown status). A maximum number of active trials is being conducted in the US and China while a minimum of trials was reported in Italy, Germany, and the UK (as represented in the graph)


Patient Advocacy Groups (PAGs) for Pancreatic Cancer
Patient advocacy groups are organizations that work to support and represent the interests of patients and their families. These groups often focus on specific medical conditions or broader healthcare issues, aiming to raise awareness, provide resources, and advocate for policies that improve patient outcomes and access to care.
Here are some of the PAGs.

References
- Cleveland Clinic
- Mayo Clinic
- Cancer.org
- NCBI
- Globe News Wire
- FDA
- EMA
- ClinicalTrials.gov
- Future Market Insights
Related Post: Disease of the Month – Ulcerative Colitis



